The PregSense Monitor: A useful new tool or fear-based marketing
By: Deena H. Blumenfeld, ERYT, RPYT, LCCE, FACCE | 0 Comments
By Deena Blumenfeld, ERYT, RPYT, LCCE
Part of a parent's job description is to worry about their children. In doing so, parents can help the child maintain their physical health and their emotional wellbeing. However, when the line is crossed into fear based parenting; they may become overprotective to the point of stifling a child's natural curiosity and the need to learn by making mistakes. They are then at risk of becoming "helicopter parents".
This is an issue of control. When parents take full control, of their child's overall well being, they feel that they are protecting them from all the negative aspects of the world. This is a fallacy.
Advertisers and marketers play into this fear and the need for control, that feeds into the parents' feelings of limited or lack of control. Companies create and market products that provide the impression of safety and security. These products provide a false sense of control for parents, which furthers the illusion that they are doing something "good" or "right" as they "protect" their baby.
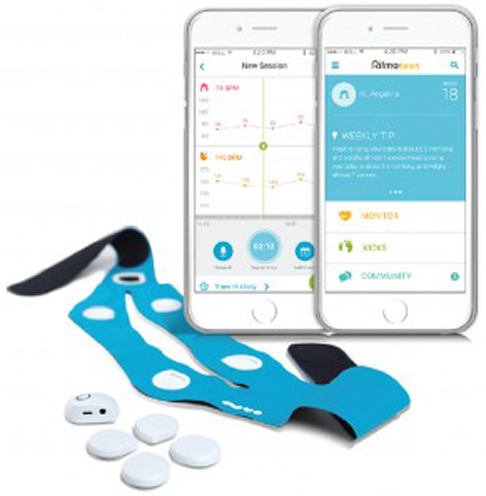
Making the rounds of Facebook, and other social media feeds, was this nifty little video about an at-home, wearable baby monitor. It's called the PregSense Monitor by Nuvo Group. The general consensus from the online community, both mothers and professionals alike, was "Wow! This is amazing! We'll save so many babies this way!"
My own reaction was a bit different. I'm a skeptic at heart and like all Lamaze educators; I'm a big fan of evidence based products, treatments, procedures and medications. So, I knew I needed to learn more about the PregSense monitor. What's the evidence behind it? Would it really meet expectations, and save babies and reduce moms' anxiety?
I attempted to contact Nuvo Group for an interview, but I have not received a response from them at the time of this writing.
Nuvo Group claims
"The Israeli tech firm hopes the device will reassure anxious mothers like Michal, in week 32 of her pregnancy, who require monitoring without having to see her doctor."
Claim: "(The monitor will) allay mothers' fears by transmitting data about the health of the mother and fetus."
- It appears to monitor all of the mother's vital signs, not unlike a Fitbit or other activity tracker. But how does having the knowledge about your own vital signs and getting additional information about baby's activities reduce fear?
- What if the monitor malfunctions? What does that do to a mother's level of fear?
- Can one make the assumption that if the monitor isn't picking up the baby, the mother will become more worried, rather than less. This might lead to increased health care provider visits and further unnecessary medical testing.
- Could wearing this monitor increase anxiety and potentially cause mothers to be so focused on the monitor it becomes a bit of an obsession?
- Mothers may become hypervigilant and reliant on the constant stream of "data" available to be reviewed.
- How would a mother feel if she was unable to wear the monitor one day? Would that increase her fears, even if those fears were unfounded?
- Removing access, even for a short time could increase worry and interefere with a mother's ability to continue her daily activities.
- When there is a constant stream of data it becomes easy to tune out the information. Wouldn't that defeat the purpose of this device?
- The information may become white noise and fade into the background, because it's a nonstop stream.
Claim: Mothers can connect, see and hear the fetus whenever they want, without needing to consult a doctor.
- Do mothers need a device to help them connect with their babies?
- This product is trying to create a consumer need that does not exist.
- Mothers connect with their babies all the time by feeling their movements; talking to them; touching their growing bellies, etc. Would the device reduce this natural mother/fetus interaction? Would a mother be more likely to turn to her smartphone for results from the monitor instead of paying attention to what her baby is actually doing throughout the rhythm of the day.?
- Using this device would require a health care provider to be monitoring all of these women, all the time. This doesn't take into account staffing levels or time to complete the task. 24/7 monitoring would be a massive time commitment and responsibility.
- What about additional liability for the health care provider for not monitoring a woman properly or correctly identifying a problem?
- We live in a very litigious society. A care provider might be facing a lawsuit if the data from the monitor is not evaluated regularly and an anomaly was missed.
- Since there are two monitor types - the clinical monitor and the consumer monitor, this raises additional questions. What if the mother is low-risk and healthy, but chooses to wear the consumer model, without a prescription to "reassure" herself that all is well?
- Would the physician then be required to monitor this mother, if there is no medical need and was not advised by the physician?
- What is the physician's liability in this case?
Claim: "We will be able to analyze this data to predict about events of pregnancy, like preterm labor, like preeclampsia and more and we will be able to intervene in the right time..."
- Preterm labor may be able to be detected with continuous monitoring. However, the monitor is only identifying contractions. It's not looking at vaginal discharge, cervical change, flu-like symptoms or downward pressure from the baby.
- Would the monitor be able to tell the difference between Braxton-Hicks contractions and early labor?
- The limited information on Nuvo Group's website and in their press release does not provide enough information to say for sure.
- What about those women who experience Braxton-Hicks regularly throughout pregnancy but are not in labor? Would the monitor be helpful or harmful for them in identifying mothers in preterm labor? Would they be in and out of their care provider's offices more frequently, causing disruption to their daily lives?
- Preeclampsia cannot be prevented at this time. So, at best, the monitor would let the mother and her care provider know that her blood pressure is high. It would not test for protein in her urine, swelling in her face, headaches, vision changes or any of the other symptoms of preeclampsia, so it's an incomplete test. Would preeclampsia be missed because mother's blood pressure is borderline and no other tests were administered.
Claim: Regarding monitoring high risk mothers with continuous monitoring in hospital; the monitor will benefit the health care provider by replacing a bulky machine with one that is lightweight and not connected to the wall.
- We already have telemetry units for Electronic Fetal Monitoring (EFM), in many hospitals. This device is now redundant and may not integrate with the current software used to monitor the EFM units.
- How much will this cost a hospital to replace all of their current EFM units by purchasing these PregSense clinical monitors? Is the financial outlay for a new convenience worth the expense?
- Does the new monitor increase safety for mother and baby in comparison to traditional EFM. Is this alternative truly better for mothers and for doctors in an in-patient setting? Where are the studies that compare the two options? Is the data we get any better? Or are we still subject to human interpretation of the data in identifying the appropriate course of action?
Claim: The PregSense monitor is safer than ultrasounds that can cause tissue damage
- From the FDA: The long-term effects of tissue heating and cavitation are not known. Therefore, ultrasound scans should be done only when there is a medical need, based on a prescription, and performed by appropriately-trained operators.
- Expectant mothers should also be aware of purchasing over-the-counter fetal heartbeat monitoring systems (also called doptones). These devices should only be used by trained health care providers when medically necessary. Use of these devices by untrained persons could expose the fetus to prolonged and unsafe energy levels, or could provide information that is interpreted incorrectly by the user.
- Without knowing what technology the PregSense monitors are using, we cannot accurately assess the risks of these devices to the fetus. Nuvo group has not fully disclosed what their technology is.
- This is not an FDA approved device (at the time of writing), therefore, we cannot be sure of its safety or its efficacy. Therefore it cannot be a recommended device. In other words, consumers should NOT be using any device at home, whether they are ultrasounds, dopplers, or other fetal heart rate monitors without a prescription from a care provider. Without FDA approval, this device may not be prescribed.. I do not anticipate that the consumer version of this product will receive FDA approval.
 At this point in time there is no evidence and no research, to support monitoring mothers at home during pregnancy. All of the literature refers to full time electronic fetal monitoring (EFM) during labor. Therefore my assumptions are based off of that literature.
At this point in time there is no evidence and no research, to support monitoring mothers at home during pregnancy. All of the literature refers to full time electronic fetal monitoring (EFM) during labor. Therefore my assumptions are based off of that literature.
Consensus among professional and governmental groups is that, based on the evidence, intermittent auscultation is safer to use in healthy women with uncomplicated pregnancies than electronic fetal monitoring (EFM). (Heelan 2013) These professional groups include ACOG and AWHONN.
The issue with the beneficial claims made by Nuvo Group is they are in opposition to what the research finds for routine continuous EFM. Continuous EFM in low risk mothers provides no benefit for babies and increases the risk of cesarean for mothers. Therefore the whole concept of the PregSense Monitor is based on an erroneous assumption. It is not possible to prevent a problem by monitoring the baby. A problem can only be detected as it is occurring. So, even if a problem is observed while doing at home monitoring, by the time the mother makes it to the hospital it is may be too late to intervene effectively.
There is also the risk of false positive results. The monitor may detect an anomaly that then increases the mother's fear about her baby's well being only to be examined to find out that her baby is doing just fine, causing undue stress and panic.
The claims of the manufacturer of this product don't hold up under current EFM guidelines and are not FDA approved.
Simplifying fetal monitoring for the care provider may not actually be the case when we look at 24/7 monitoring which still needs to be interpreted by a human being and a potentially large financial investment for a hospital that already has an EFM system that is adequate.
The claim that this product is safer than what currently exists with today's EFM technology and ultrasonography is unsubstantiated. Without proper research, we do not know if it is safer, more harmful or neutral in relation to EFM and ultrasound as they are done today.
Resolving mother's fears and helping her connect with the baby are at best an assumption regarding the "softer side" of the product's results. It may be that some women do have greater piece of mind and feel a greater connection with their baby when using the device. Selling a feeling does not provide medical benefit to mother or baby. It is, however, good marketing.
The takeaway for your students is to have them look at all products with a discerning eye. Fear based marketing is insidious and plays to their emotions. They need to be making informed decisions based on accurate and evidence based information, rather than an emotional response to something that hits them in the heart.
References:
Nuvo Group's website
Reuters, "Wearable device provides continuous fetal monitoring"
Dekker, Rebecca, Evidence Based Fetal Monitoring, 2012
Dekker, Rebecca, What is the Evidence for Fetal Monitoring on Admission, 2012
FDA, Avoid Fetal "Keepsake" Images, Heartbeat Monitors, 2014
FDA, Ultrasound Imaging
ACOG Practice Bulletin #106, "Intrapartum Fetal Heart Rate Monitoring: Nomenclature, Interpretation, and General Management Principles,", July 2009
ACOG press release, ACOG Refines Fetal Heart Rate Monitoring Guidelines, 2009
Lisa Heelan, MSN, FNP-BC, Fetal Monitoring: Creating a Culture of Safety With Informed Choice, J Perinat Educ. 2013 Summer; 22(3): 156-165.
Published: August 17, 2015
Tags
Electronic Fetal MonitoringDeena BlumenfeldLabor/BirthMaternal Infant CareDoulasFetal Monitoringchildbirth educatorPregSenseSpeech Therapist