The Healthy Birth: Dyad or Triad? Exploring Birth and the Microbiome
By: Sharon Muza, BS, LCCE, FACCE, CD/BDT(DONA), CLE | 0 Comments
By Anne Estes, PhD, Illustrated by Cara Gibson, PhD
There has been much discussion and burgeoning research on how the mode of birth affects the microbiome of the infant (and later on the adult). It is becoming clear that how babies are born impacts the type of bacteria that take up residence in and on our bodies. Today, I would like to welcome researcher and writer Anne Estes, PhD, and researcher and illustrator Cara Gibson, PhD to Science & Sensibility. Anne shares information on the research into a newborn's (and later on the adult) microbiome and how it can be affected by the location of birth, the type of birth and the interventions that occur during birth. Learn more about what this new field of research is telling us about the importance of the microbiome. Stay tuned for a future interview by Anne, with some of the research scientists attempting to supplement the microbiome of infants delivered by planned Cesareans. - Sharon Muza, Science & Sensibility Community Manager
Birth plans often change. Neither my husband nor I anticipated the series of interventions with my first daughter's birth. In the end, though we had the most important outcome - a healthy mom and baby dyad. How did these interventions influence the health of the third, silent, and invisible member of my daughter's birth that I hadn't included in her birth plan - her microbiome?
The helpful and harmful bacteria, viruses, and fungi that live in and on every environment, both living and non-living, are the microbiome of that environment. The bacterial component of the microbiome is best understood to date and will be this post's focus. An organism's microbiome influences the development and health of those animals and plants, whereas the microbiome of soil and buildings influence organisms that reside in those non-living environments. Our helpful microbes provide services that range from vitamin synthesis and food degradation to preventing attacks by pathogens. However, in the last few centuries of human-microbe interactions, changes in our birth and medical practices and living conditions may have altered the acquisition of our microbial communities. Our altered microbiomes, especially in the industrialized world, may help explain the increase in allergies, asthma, diabetes, gastrointestinal diseases, and mental disorders, such as depression, anxiety, and autism.
Humans as ecosystems for microbes
To a bacterium, you are a planet made up of several different ecosystems. From the dry, UV-intense "desert" of your skin to the warm, wet, nutrient-rich "lake" of your mouth, specific bacteria live in different regions on a person, just as specific vertebrate animals live in different ecosystems on the Earth (Figure 1, left and center). As ecosystems of the human environment change during development, pregnancy, or with changing diets, which bacterial species remain or how these microbial species function may shift is slowly becoming understood. How do we first acquire these microbes? Previous posts here and other blogs have done excellent reviews of the human microbiome and birth, so my post will serve to provide updates and pose new questions for consideration.
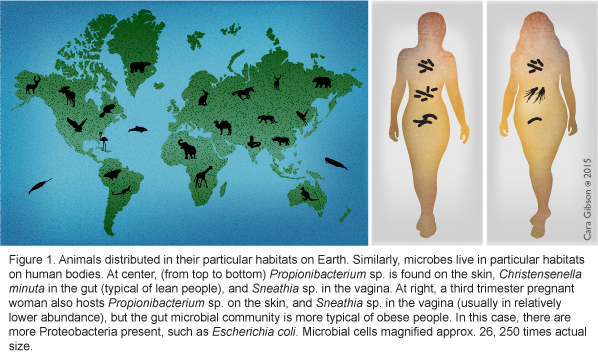
The source of the infant microbiome
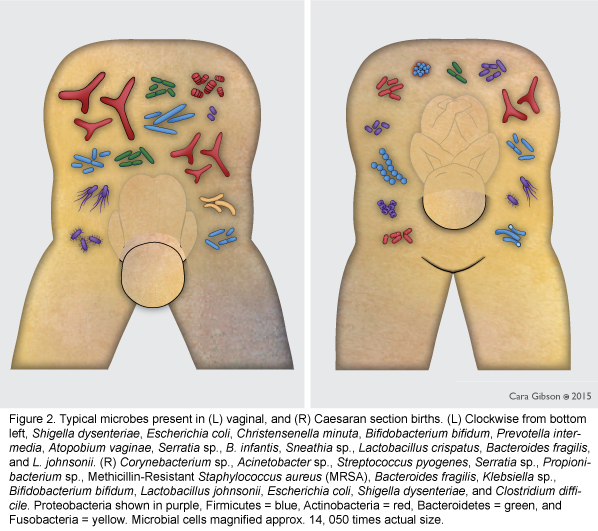
The infant microbiome is acquired during birth [1, 2], from first foods [3-5], and the environment [6], and may also be partially colonized in utero [7]. The microbiome of infants born vaginally most closely represents the microbiome of the mother's vagina and feces [1], and is rich in beneficial bacteria such as Bifidobacterium longum subsp. infantis and Bacteroidetes [8, 9] (Figure 2, left). In contrast, the microbiome of infants born via planned Cesarean is more similar to that of the mother's skin and hospital environment [1]. The microbiomes of planned Cesarean-born infants are more likely to have hospital-acquired pathogens such as Clostridium difficile, methicillin-resistant Staphylococcus aureus (MRSA), and pathogenic Es. coli [1] and lack beneficial Bacteroidetes and Bi. longum subsp. infantis [10] (Figure 2, right). However, when beneficial Bifidobacterium were occasionally present in Cesarean-born infants, pathogenic Es. coli and C. difficile were not found [11] suggesting that one benefit of Bifidobacterium, especially Bi. longum subsp. infantis, may be outcompeting these potential pathogens.
Influence of birth mode on microbiome transmission
Repeatedly, studies in different countries, ethnic groups, ages, and health status have suggested that planned Cesarean-born infants are more likely to have more health issues and a different microbiome, as compared to vaginally born infants [2, 10, 12-14]. These differences in community composition can even be seen in adulthood [15]. A new Canadian study finds that the microbiome of infants born via unplanned Cesarean had increased bacterial richness and diversity, more similar to that of vaginally born infants than planned Cesarean [10]. Unfortunately, this was only a small study where fewer than ten mother-infant pairs were examined. Several variables such as length of time in labor or how far labor progressed, antibiotic use, natural vs. artificial rupture of membranes, and/or other interventions that may influence the microbiome were also not examined [10]. However, it does suggest that the process of labor, perhaps the hormonal or other physiological changes, may influence the microbiome. Additionally, some maternal bacteria may be transmitted when membranes rupture during labor [10]. Are bacteria "eavesdropping" on the chemical changes in the human to prepare themselves for transmission to the baby? Do these maternal hormone changes lead to increased vaginal or gut epithelial sloughing to transmit more or specific bacteria? Certainly, studies with larger sample sizes that can help control for these variables along with experimental studies on model animals are warranted.
Influence of birth place on microbiome transmission
Infants also acquire a proportion of their microbiome from their physical, inanimate surroundings. What proportion of the microbiome and which bacteria are acquired most likely depends on how many and what kinds of bacteria are acquired in utero, through birth method, and first foods. Since Cesarean delivered infants seem to be exposed to a lower density of maternal bacteria than vaginally delivered babies, the former may be more likely to acquire bacteria from their environment. However, this hypothesis has not been examined.
Just as living organisms are a microbial environment, so are non-living structures such as buildings. Scientists at several universities working together on The Microbiology of the Built Environment Project funded by the Sloan Foundation are comparing the microbes of homes and hospitals. They have found that buildings are quickly colonized by the microbes of the people living in them [16]. Such rapid colonization specific to the individual being housed is even seen in infants in the neonatal intensive care unit (NICU) [17, 18] (Figure 3).

One group is surveying the microbiome of a hospital over time, as it is being built and then occupied. Hospital-acquired infections are an increasing concern for all patients, especially newborns. Infants born by Cesarean have an increased rate of MRSA, C. difficile, and other opportunistic pathogens [1]. However, different hospitals and even wards within a hospital might be expected to have disparate levels of pathogens depending on how prevalent the disease is within the hospital patients and staff. Whether freestanding birth centers, operating rooms dedicated to labor-and-delivery only, and mixed-use operating rooms have dissimilar microbiomes has yet to be investigated. Infants born in private homes would be exposed to the same microbiomes of members of the household.
Influence of first foods on microbiome transmission
First foods are another influence on the infant microbiome. Breastfed infants have two "moms" their human mother and their Milk-Oriented Microbiota (MOM) (Figure 4, left). The MOM are beneficial, protective bacteria in the infant's gut that thrive when fed the sugars in breast milk [19]. Although human milk oligosaccharides (HMOs) are the third most abundant component of breast milk, the infant cannot digest these sugars. Instead, HMOs are a natural prebiotic or "bacterial food". Various HMO sugar types and concentrations influence bacterial diversity, keeping strains of Bifidobacterium longum subsp infantis in highest abundance in the first few months of life and preventing pathogens from binding to the gut [20]. HMOs vary between pre-term and full term birth, vaginal deliveries and planned Cesarean births (reviewed in [20]), and even between mothers with different types of "secretor" genes [21]. Does this HMO variability serve to maintain and enhance some of the differences in bacterial communities between individuals? In addition to the MOM, a diversity and abundance of bacteria are found in breast milk. The average breastfed baby is exposed to between 1 and 10 million bacteria daily from their mother's milk [5]! The breast milk microbiome is a unique assemblage of bacteria, distinct from human skin, gut, oral, vaginal, and other specific body site microbiomes [4]. Like other components of breast milk, the bacterial community changes dramatically between colostrum and mature milk with colostrum being the most diverse with over 1,000 different bacterial types [4]. Although only ten women were followed, it is intriguing that the breast milk microbiome of women delivering via planned Cesarean at birth, one month, and six months post-birth, was more similar to their gut microbiome than the breast milk of mothers who delivered vaginally [4]. Milk of moms undergoing unplanned Cesarean and vaginally delivering mothers were most similar [4]. How the presence of different microbes influences the developing human infant immune system has yet to be determined. Additionally, does the breast milk bacteria colonize the infant gut or are they digested? Could breast milk bacteria change how the MOM infant gut microbiome works as they pass through the gut, as one probiotic does in elderly patients [22]?
Formula-fed babies have a more diverse and rich microbiome than breast-fed babies, with lower numbers of Bifidobacterium and higher abundances of Peptostreptococcaceae, which includes C. difficile [10, 23] (Figure 4, right). Gut bacterial diversity is essential in increasing the ability of adults to digest a wide variety of foods. However, bacterial diversity may be detrimental in the infant stage when the immune system is developing and learning to distinguish between microbes that are friends and those that are foes. Breast milk sugars may mediate the relative abundances of different bacterial species [24]. Through studies like the Milk Bioactives Program at University of California at Davis, more is learned about the interaction between breast milk sugars and specific bacteria that can lead to better probiotic and prebiotic formulas and improve infant health.
Influence of in utero environment on microbiome transmission
Many other factors surrounding birth may influence the infant microbiome. High levels of reported maternal stress and high cortisol concentrations during pregnancy, correlated with lower relative abundances of beneficial Lactobacillus and Bifidobacterium sp. and higher abundances of Proteobacteria, such as Enterobacter and Escherichia. Infants of these highly stressed mothers had increased reports of gastrointestinal symptoms and allergic reactions, though these issues were reported by caregivers, not physicians, which may confound the findings [25]. A separate study found infants whose gestation lengths were less than 38 weeks had microbiome communities that were low in Bifidobacterium and took 3 to 6 months to reach a normal Bifidiobacterium-rich community as compared to infants born at 40 or more weeks [9]. Finally, the use of antibiotics during pregnancy [12] may also lead to infant health issues.
Do birth interventions change the microbiome?
The potential "eavesdropping" of bacteria on human hormones during pregnancy and labor led me to wonder how the use of synthetic hormones such as Pitocin, especially during stalled labor, might influence the microbiome and overall infant health. There are so many variables to the birth process that many of these questions could only be answered with extremely detailed data of tens of thousands of mother-infant-microbiome triads over time. The influence of interventions such as epidurals, frequency of cervical checks, episiotomies, vaginal preparation with betadine, enemas, and other procedures used during labor and delivery also have not been extensively examined. In general, any procedure that "sterilizes" or cleans the vaginal and rectal area would most likely decrease the transmission of the mother's microbial community. Whether cervical checks introduce skin or environmental microbes to the infant should also be considered. Finally, what effect does postponing baby's first bath until 24 or 48 hours after birth have on microbial colonization? What role does the vernix have in facilitating the colonization of the infant's microbiome?
From lab bench to birth room
Antibiotics, Cesarean delivery, and other interventions are valuable and life-saving for many women and infants; however, as they have become more commonly used we have seen an increase in many long-term diseases and disorders. Recent microbiome research suggests that we should consider birth as delivering and nurturing a healthy triad - mom, infant, and microbiome. Currently, studies are being conducted to swab Cesarean delivered infants with vaginal secretions immediately after birth. Should fecal microbiome members also be considered? If hormone surges are important for the microbiome transmission during labor and in breast milk, as the unplanned Cesarean data suggest, how could the natural hormone surges of labor be mimicked for planned Cesarean? When antibiotics are needed for mother or infant, how best can we quickly repopulate the disturbance to the native microbiome?
Humans, and all organisms, are planets with diverse ecosystems. In sequencing of the human genome, we learned that diseases rarely correlated to specific human genes. Most likely instead of focusing on only the human or only the microbes, we should be examining the intersection between human genomics and microbiome structure and function to best understand health and disease of human-microbe ecosystems. Both human genomics and microbiome work are in their infancy (pun intended). Researchers examine correlations to develop testable hypotheses that can be examined in non-human animal models. Yet many of the microbes of interest are currently unable to be cultivated for direct testing or probiotic use. At this time, directly translating research findings to the delivery room is difficult, but I hope that this post will stimulate thought and conversations about the silent, invisible, yet important third member of human birth and life.
References
- Dominguez-Bello, M. G., E. K. Costello, M. Contreras, M. Magris, G. Hidalgo, N. Fierer, and R. Knight. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences 107:11971-11975.
- Mueller, N. T., E. Bakacs, J. Combellick, Z. Grigoryan, and M. G. Dominguez-Bello. 2015. The infant microbiome development: mom matters. Trends in Molecular Medicine 21:109-117.
- Zivkovic, A. M., J. B. German, C. B. Lebrilla, and D. A. Mills. 2011. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proceedings of the National Academy of Sciences 108:4653-4658.
- Cabrera-Rubio, R., M. C. Collado, K. Laitinen, S. Salminen, E. Isolauri, and A. Mira. 2012. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. The American Journal of Clinical Nutrition 96:544-551.
- Fernández, L., S. Langa, V. MartÃn, A. Maldonado, E. Jiménez, R. MartÃn, and J. M. RodrÃguez. 2013. The human milk microbiota: Origin and potential roles in health and disease. Pharmacological Research 69:1-10.
- Thompson, A. L., A. Monteagudo-Mera, M. B. Cadenas, M. L. Lampl, and M. A. Azcarate-Peril. 2015. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Frontiers in Cellular and Infection Microbiology 5.
- Prince, A. L., D. M. Chu, M. D. Seferovic, K. M. Antony, J. Ma, and K. M. Aagaard. 2015. The Perinatal Microbiome and Pregnancy: Moving Beyond the Vaginal Microbiome. Cold Spring Harbor Perspectives in Medicine.
- Jost, T., C. Lacroix, C. P. Braegger, and C. Chassard. 2012. New Insights in Gut Microbiota Establishment in Healthy Breast Fed Neonates. PLoS ONE 7:e44595.
- Dogra, S., O. Sakwinska, S.-E. Soh, C. Ngom-Bru, W. M. Brück, B. Berger, H. Brüssow, Y. S. Lee, F. Yap, Y.-S. Chong, et al. 2015. Dynamics of Infant Gut Microbiota Are Influenced by Delivery Mode and Gestational Duration and Are Associated with Subsequent Adiposity. mBio 6.
- Azad, M. B., T. Konya, H. Maughan, D. S. Guttman, C. J. Field, R. S. Chari, M. R. Sears, A. B. Becker, J. A. Scott, and A. L. Kozyrskyj. 2013. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Canadian Medical Association Journal 185:385-394.
- Musilova, S., V. Rada, E. Vlkova, V. Bunesova, and J. Nevoral. 2015. Colonisation of the gut by bifidobacteria is much more common in vaginal deliveries than Caesarean sections. Acta Paediatrica 104:e184-e186.
- Mueller, N. T., R. Whyatt, L. Hoepner, S. Oberfield, M. G. Dominguez-Bello, E. M. Widen, A. Hassoun, F. Perera, and A. Rundle. 2014. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes.
- Neu, J., and J. Rushing. 2011. Cesarean versus Vaginal Delivery: Long term infant outcomes and the Hygiene Hypothesis. Clinics in perinatology 38:321-331.
- van Nimwegen, F. A., J. Penders, E. E. Stobberingh, D. S. Postma, G. H. Koppelman, M. Kerkhof, N. E. Reijmerink, E. Dompeling, P. A. van den Brandt, I. Ferreira, et al. 2011. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol 128:948-55 e1-3.
- Goedert, J. J., X. Hua, G. Yu, and J. Shi. 2014. Diversity and Composition of the Adult Fecal Microbiome Associated with History of Cesarean Birth or Appendectomy: Analysis of the American Gut Project. EBioMedicine 1:167-172.
- Lax, S., D. P. Smith, J. Hampton-Marcell, S. M. Owens, K. M. Handley, N. M. Scott, S. M. Gibbons, P. Larsen, B. D. Shogan, S. Weiss, et al. 2014. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345:1048-1052.
- Brooks, B., B. Firek, C. Miller, I. Sharon, B. Thomas, R. Baker, M. Morowitz, and J. Banfield. 2014. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2:1.
- Raveh-Sadka, T., B. C. Thomas, A. Singh, B. Firek, B. Brooks, C. J. Castelle, I. Sharon, R. Baker, M. Good, M. J. Morowitz, et al. 2015. Gut bacteria are rarely shared by co-hospitalized premature infants, regardless of necrotizing enterocolitis development, vol. 4.
- Zivkovic, A. M., Z. T. Lewis, J. B. German, and D. A. Mills. 2013. Establishment of a Milk-Oriented Microbiota (MOM) in Early Life: How Babies Meet Their Moms. Functional Food Reviews 5:3-12.
- Smilowitz, J. T., C. B. Lebrilla, D. A. Mills, J. B. German, and S. L. Freeman. 2014. Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annual Review of Nutrition 34:143-169.
- Lewis, Z., S. Totten, J. Smilowitz, M. Popovic, E. Parker, D. Lemay, M. Van Tassell, M. Miller, Y.-S. Jin, J. German, et al. 2015. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 3:13.
- Eloe-Fadrosh, E. A., A. Brady, J. Crabtree, E. F. Drabek, B. Ma, A. Mahurkar, J. Ravel, M. Haverkamp, A.-M. Fiorino, C. Botelho, et al. 2015. Functional Dynamics of the Gut Microbiome in Elderly People during Probiotic Consumption. mBio 6.
- Bezirtzoglou, E., A. Tsiotsias, and G. W. Welling. 2011. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 17:478-482.
- Guaraldi, F., and G. Salvatori. 2012. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Frontiers in Cellular and Infection Microbiology 2:94.
- Zijlmans, M. A. C., K. Korpela, J. M. Riksen-Walraven, W. M. de Vos, and C. de Weerth. 2015. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53:233-245.
About Anne Estes

Anne M. Estes, PhD is a postdoctoral fellow at the Institute for Genome Sciences in Baltimore, MD. She is interested in how microbes and their host organisms work together throughout host development. Anne blogs about the importance of microbes, especially during pregnancy, birth, first foods, and early childhood at Mostly Microbes.
About Cara Gibson

Cara Gibson, BSc (Hon), MS, PhD was trained as an entomologist (insect scientist) and her interests include ecology, biodiversity, and interactions with microbial symbionts. She has worked as a field ecologist, research scientist, educator, outreach coordinator, and scientific illustrator. Dr. Gibson would like to help bridge the gap between current practices and new research to improve women's health and birth outcomes. Contact Cara at caramgibson at gmail dot com for illustration inquiries / permissions.
Published: April 27, 2015
Tags
BreastfeedingCesareanMicrobiomeProfessional ResourcesLabor/BirthMaternal Infant CareBacteriaImproving teaching skillsPractical skillsbetter birth outcomesentrepreneurAnne EstesCara Gibson