A Health Care Provider's Guide to Newborn Screening
By: Sharon Muza, BS, LCCE, FACCE, CD/BDT(DONA), CLE | 0 Comments
By Elizabeth Jones, APHL
Today, Elizabeth Jones brings us up to date on the current research and information we need to know in order to be able to accurately discuss newborn screening and the research behind it with our clients, students and patients. Make sure you are current on what you need to know to be able to share with families. On Thursday, we will hear from some families whose lives were changed by this important newborn screening. - Sharon Muza, Community Manager.
By U.S. Air Force photo/Staff Sgt Eric T. Sheler
(USAF Photographic Archives via Wikimedia
Commons
Newborn screening was recently named one of the Top 10 Great Public Health Achievements by the Centers for Disease Control and Prevention (CDC, 2011). These simple tests save or improve the lives of approximately 12,000 babies in the U.S. every year (CDC, 2012).
For babies who test positive for one of the newborn screening conditions, rapid identification and treatment makes the difference between health and disability, or even life and death. Yet most parents don't know about newborn screening. It is crucial for providers to have the answers that can help parents understand the importance of newborn screening and rapid follow-up action if a baby screens positive for a newborn screening condition.
What is newborn screening?
Newborn screening is the practice of testing every baby within the first two days of life for certain rare but harmful or potentially fatal genetic and metabolic conditions that are not otherwise apparent at birth. Early identification of these conditions in newborns facilitates timely intervention(s) that result in significant decreases in morbidity, mortality and disability (Lloyd-Puryear, 2006).
What are some examples of newborn screening conditions?
Most newborn screening conditions are metabolic and genetic disorders that may be inherited or appear spontaneously . Examples of newborn screening conditions are MCAD - a disease that results in the build-up of fatty acids in the body and can lead to brain damage and breathing problems; cystic fibrosis - a disease that affects the lungs, pancreas and digestive system; congenital hypothyroidism - a disease where infants are missing their thyroid gland or do not produce enough of a particular thyroid hormone which stimulates growth and brain development; and sickle cell anemia - a disease where the red blood cells have an abnormal shape reducing their ability to carry oxygen throughout the body. (HRSA, 2013).
How is newborn screening done?
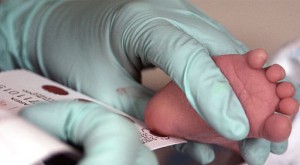
Screening begins by pricking a newborn's heel to get enough blood to fill a few circles on a filter paper card. The specimen(s) are then sent to a state-designated newborn screening laboratory for analysis. Newborn screening goes beyond blood spot screening to include point-of-care testing for hearing and, in some states, for critical congenital heart disease. Hearing is evaluated using either an otoacoustic emissions test or auditory brain stem response. Critical congenital heart disease screening is performed using pulse oximetry to measure blood oxygen levels. These noninvasive tests are performed shortly after birth and follow-up is conducted by the state newborn screening program (Baby's First Test, 2013).
When does newborn screening take place?
The specimen is collected by a healthcare provider, typically at the birthing location, during the first 24 to 48 hours of life. After birth, infants need approximately 24 hours of metabolizing food before a reliable specimen can be obtained. The recommendation to test within 48 hours relates to the length of a hospital stay for a typical delivery. Screening can be done a bit later, but it is recommended that it is completed as soon as possible for optimal intervention if necessary. Some states require two specimens to be collected, in which case it is recommended the second be collected between seven and 15 days of life (Oregon Health and Sciences University, 2010).
Why is this time period important?
Most newborn screening disorders can cause death or disability in the first weeks of life so it is important to obtain a specimen shortly after birth but prior to discharge from care to avoid missing a child with a disorder. This quick turnaround also allows for prompt follow-up and treatment if necessary. Some states obtain a second specimen because there are a few disorders, such as endocrine disorders, which may not be easily detected until the second week of life due to natural fluctuations in the analytes used to measure these disorders (Oregon Health and Sciences University, 2010).
What happens when a test is out of normal range?
When a test result is out of normal range, laboratory or follow-up personnel contact the birthing facility and the newborn's physician to ensure that the child receives the appropriate diagnostic work-up and treatment. In some states, follow-up personnel may contact the family directly. It is crucial that providers and parents take follow-up calls very seriously and follow recommendations closely. Many conditions can cause babies to go into distress without warning.
Why do some babies screen as falsepositive for a condition?
False-positives are an unfortunate product of ensuring that the highest number of babies with disorders are detected; preventing false-negatives is of utmost importance (Koepsell, 2003).
They are uncommon, but they do occur. Newborn screening is not a diagnostic test, which is why follow-up and confirmatory testing are critical.
Can a parent decline newborn screening?
Refusing newborn screening is strongly discouraged as many newborn screening conditions have severe, irreversible and rapid consequences when undetected and left untreated. Many state newborn screening programs have an opt-out policy, requiring testing for all newborns unless parents or guardians decline testing due to religious or other reasons (Therrell, 2003). Not all states allow parents to opt out. For more information on your state's opt-out policy, please contact your state's department of health.
Are there national recommendations regarding newborn screening?
The Discretionary's Advisory Committee on Heritable Disorders in Newborns and Children (DACHDNC; formerly known as the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children) works with the US Secretary of Health and Human Services to develop a core panel of conditions, now called the Recommended Universal Screening Panel (RUSP). Currently the group recommends screening for 31 core conditions, which includes hearing and critical congenital heart disease, as well as 26 secondary conditions/targets which could be identified during the screening process. Each state determines which conditions it will include on its newborn screening panel. Most states screen for at least 29 core conditions and most of the secondary conditions/targets on the RUSP. Visit BabysFirstTest.org or NewSTEPs.org to find out which conditions are included on your state's newborn screening panel.
What technologies are used for screening?
Laboratory tests used to detect newborn screening disorders include colorimetric and fluorometric immunoassays, isoelectric focusing, high-performance liquid chromotagraphy, tandem mass spectrometry, and molecular testing/DNA based tests. The introduction of tandem mass spectrometry into newborn screening in the 1990s allowed simultaneous testing of an array of metabolic conditions using a single 3 mm size specimen punched from a dried blood spot (Chace, 2003).
How is molecular testing used in newborn screening?
Molecular testing can increase the speed of diagnosis and treatment and reduce the number of false positives (Zhang 1994). Primarily used as a second-tier test for conditions such as cystic fibrosis, molecular testing allows for differentiation between specific disorders such as sickle cell anemia and sickle/beta-thalassemia (Clarke, 2000). Severe Combined Immunodeficiency was the first newborn screening disorder to use molecular technology as the primary method of screening (Baker, 2009).
What resources are available to educate parents and health partners about newborn screening?
References
Baby's First Test: How screening works. Available from: URL: http://www.babysfirsttest.org.
Baker MW, Grossman WJ, Laessig RH, Hoffman JL, Brokopp CD, Kurtycz DF, et al. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol 2009;124:522-7.
Centers for Disease Control and Prevention. CDC Grand Rounds: Newborn Screening and Improved Outcomes. MMWR Morb Mortal Wkly Rep. 2012;61 (No. 21): 390-393.
Centers for Disease Control and Prevention. Ten great public health achievements - United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60 (No. 19): 605-644.
Centers for Disease Control and Prevention (US). Newborn Screening Quality Assurance Program. Available from: URL: http://www.cdc.gov/labstandards/nsqap.html.
Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem 2003;49:1797-817.
Clarke GM, Higgins TN. Laboratory investigation of hemoglobinopathies and thalassemias: review and update. Clin Chem 2000;46(8 Pt 2):1284-90.
Health Resources and Services Administration (US). Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Newborn Screening: Toward a Uniform Screening Panel and System. Available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/. Accessed July 10, 2013.
Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness. Oxford, England Oxford University Press 2003.
Lloyd-Puryear MA, Tonniges T, Van Dyck PC. et al. American Academy of Pediatrics Newborn Screening Task Force recommendations: how far have we come? Pediatrics 2006;117(5 Pt 2):S194-211.
Oregon Health and Science University. Oregon Practitioner's Manual. 9th Edition. 2010.
Therrell BL Jr. Ethical, legal, and social issues in newborn screening in the United States. Southeast Asian J Trop Med Public Health 2003;34 Suppl 3:52-8.
Zhang YH, McCabe LL, Wilborn M, Therrell BL Jr, McCabe ER. Application of molecular genetics in public health: improved follow-up in a neonatal hemoglobinopathy screening program. Biochem Med Metab Biol 1994;52:27-35.
About Elizabeth Jones
 Elizabeth Jones, MPH, is the Newborn Screening and Genetics Senior Specialist at the Association of Public Health Laboratories (APHL). As a Senior Specialist, Elizabeth works closely with member state public health laboratories and federal, local and private partners to achieve newborn screening and genetics objectives. Her work has involved managing newborn screening projects, coordinating education and outreach activities, developing policy statements, leading quality improvement initiatives for newborn screening systems, providing information and resources to laboratories, and planning workshops, trainings, and the Newborn Screening and Genetic Testing Symposium for newborn screening stakeholders. Elizabeth holds a Master's in Public Health in Epidemiology from the State University of New York at Albany.
Elizabeth Jones, MPH, is the Newborn Screening and Genetics Senior Specialist at the Association of Public Health Laboratories (APHL). As a Senior Specialist, Elizabeth works closely with member state public health laboratories and federal, local and private partners to achieve newborn screening and genetics objectives. Her work has involved managing newborn screening projects, coordinating education and outreach activities, developing policy statements, leading quality improvement initiatives for newborn screening systems, providing information and resources to laboratories, and planning workshops, trainings, and the Newborn Screening and Genetic Testing Symposium for newborn screening stakeholders. Elizabeth holds a Master's in Public Health in Epidemiology from the State University of New York at Albany.
Published: July 15, 2013
Tags
Childbirth educationInformed ConsentCDCNewborn ScreeningMaternal Infant CareBabiesGuest PostsElizabeth JonesNewborn TestingPKU